Every day is a new opportunity to create a healthier world.
If you are reading this, I firstly want to say it’s a pleasure to meet you and I’m very thankful for your interest to integrate advances in data science and technology with human science expertise to help people in healthcare make better decisions and advance human health. We are extremely passionate about rare diseases and the ability to make the world a better place for patients and families suffering with rare diseases especially haematological diseases.
Over 60 million patients in the UK, Europe and the US suffer with some form of rare disease, with a further 340 million or more seen across the rest of the globe. 1 in 17 individuals are expected to have a rare disease at some point in their lives, 69.9% of these patients seeing an onset of the disease exclusively in childhood, and the last decade has seen a high prevalence of diseases that are primarily haematological, CNS, or with classification as a rare cancer – although numerous diseases impact multiple systems within the body.
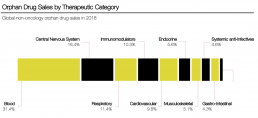
For the over 400 million patients worldwide impacted by more than 7,000 identified rare diseases, 80% of these with a genetic origin, the journey to seek the care they truly need to manage their disease effectively and maintain a good quality of life is severely hindered by the very nature of their disease, and the subsequent knowledge base around them. At its core, clinician understanding of such conditions is incredibly limited – 40% of general practitioners and 24% of specialist doctors report they simply do not have the time to work on these diagnoses. Coupled with the vast, overlapping spectrum of signs and symptoms associated with many rare diseases – the lack of an in-depth or accurate natural history that can be derived, due to a smaller patient cohort, making specific pinpointing of the disease or genotype difficult – misdiagnosis is subsequently all too common.
We have a number of programmes currently being progressed in the rare disease space and would love to have you join us as part of a small team of hard working, talented, committed and highly skilled individuals. Fortunately for us we have years of experience in data, healthcare and a great understanding of some of the diseases we are focused on. What we need from you are the things which we are not so good at yet, which are a very specific set of skills primarily focused on supporting us in the following areas.
Job Title: Real World Evidence Clinical Research
Manager Location: Remote working
Job Type: Full-time
Background
Real world evidence (RWE) is now important to support regulatory approval and to bridge the gap in evidence needs between regulators (Phase II and III) and different health technology and payer archetypes. Conditional approvals and cost justification are the norm, and the generation of sufficient, robust RWE is a key driver in maximising revenue for our pharmaceutical clients.
RWE is also used to ensure optimum resource utilisation, patient flow dynamics, biomarker collection, future clinical trial design and many more applications. RWE is generated in various ways such as chart reviews, medical record checking, new technology such as devices, application of data, and more.
Draper & Dash (D&D) utilises large data platforms with AI and machine learning capabilities, embedded within healthcare provider systems to generate RWE for multiple reasons for our biopharma clients.
As part of this process, D&D works closely with clients to design the most appropriate RWE protocols, to ensure that the capabilities of the data platforms are fully utilised to answer the client’s data gaps and to guide pre-clinical and clinical development programme design and decision-making.
Main Responsibilities
- Fully understand and convey to clients the capabilities of the D&D platform in a RWE context
- Work with biopharma clients to design and execute RWE protocols across a wide range of therapy areas
- Prepare project plans, governance processes, milestone events, project tracking and reporting to clients and D&D
- Project manage the entire process, providing the link between clients and D&D technical teams
- Provide the essential link between healthcare providers and D&D, and manage the information governance (IG) process which allows access to the data
- Lead the writing of study protocols, statistical analysis plans and study reports
- Provide methodological insights and senior review in support of various study documents
- Provide all aspects of data presentation – abstracts, manuscripts, presentations to conferences and more
- Develop the client relationship to encourage new business opportunities, including proposal writing
Qualifications and Strengths
- MSc or PhD (or an equivalent degree) in biosciences, epidemiology, public health, biostatistics, bioinformatics or a related discipline
- 3 years + work experience in real world evidence for the biopharma industry, a CRO, healthcare provider, HTA, or leading related academic institution
- Some experience in prospective or retrospective RWE evidence generation using anonymised patient level databases
- Empathetic and effective collaborative working with internal and external stakeholders
- Effective verbal and written English skills are essential
- Management of short deadlines and client needs is important, along with ensuring high quality professional deliverables
About Us
Draper & Dash Healthcare (D&D) is a London based Venture Capitalist (VC) backed, healthcare AI and Machine Learning, predictive data and analytics company. We leverage data from the public sector and private healthcare companies both nationally and globally, providing healthcare and human science organisations with solutions that improve quality, safety, outcomes, efficiencies and opportunities.
Over the years, we have worked with over 70 healthcare providers in the UK, Middle East and Australia, helping them to drive cost improvements, form new strategic partnerships, improve health outcomes and more.
Our approach integrates advances in data science and technology with human science expertise to help people in healthcare make better decisions and advance human health. D&D Healthcare works closely with treatment centres and academic bodies to support patients and industry in moving forward rapid ML and AI driven patient identification, diagnosis and access to rare treatment options. We are focused on predicting clinical events, improvement in patient management, therapeutics treatment impact.
Together, our partners help us to form some of the largest and highest-quality source for real-world evidence – including both structured and unstructured data, and longitudinal clinic data with diseasespecific models that evolve with the standard of care. We are committed to advancing human health and delivering real value and outcomes for customers and patients.